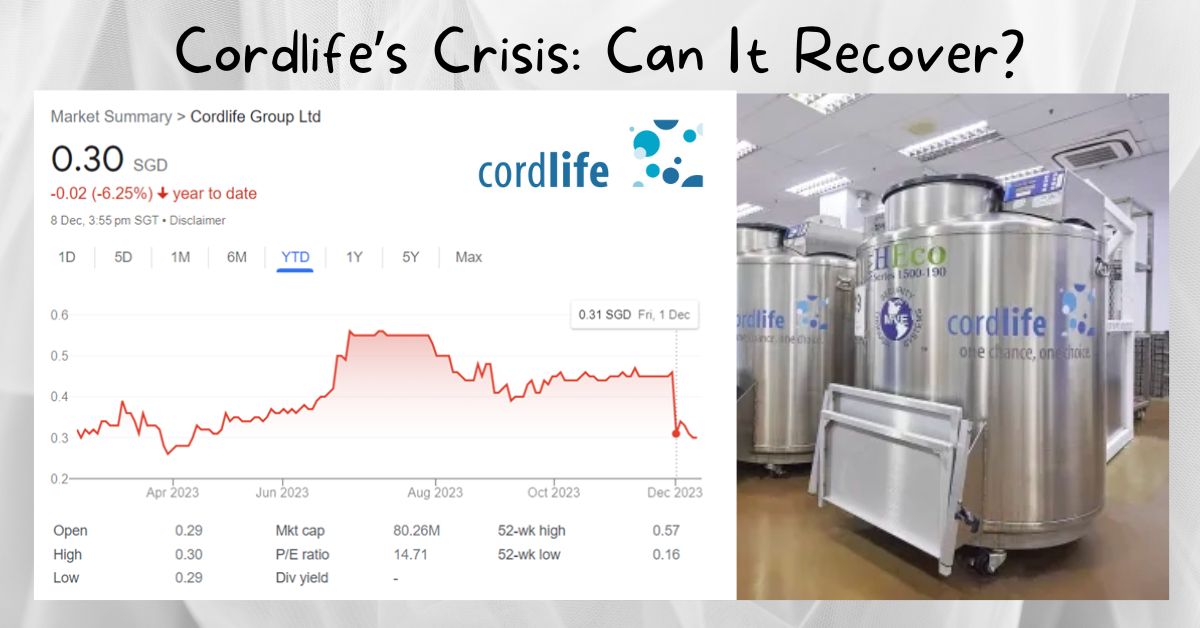
Recent events at Cordlife Group Ltd have made quite a stir, grabbing the attention of parents and investors alike. Cordlife, well-known for its specialized services in stem cell preservation, unfortunately encountered a significant operational slip-up that led to the damage of cord blood units for approximately 2,150 families.
This incident isn’t just a minor blip on the radar; it raises concerns regarding the company’s operational integrity. To add to the woes, on December 1st, following the news of this misstep, Cordlife’s shares took a substantial tumble, plummeting by over 30%.
With that said, this article will delve into the consequences of this event on Cordlife’s share price and the potential long-term effects it might have on the company.

The Incident Unfolds
The incident at Cordlife Group Ltd began with a public tip-off on July 24, alleging that some cord blood units stored by the company were exposed to temperatures above 0 degrees Celsius, alongside other quality concerns. This tip triggered the Ministry of Health (MOH) into action, prompting a series of surprise audits, with the earliest one dated back to August 15th.
What these investigations unveiled was quite concerning. Seven of Cordlife’s cryogenic storage tanks had been experiencing temperature irregularities, deviating from the normal temperature ranges. These irregularities had been going on since November 2020, potentially jeopardizing the viability of the cord blood units (CBUs) stored in those tanks.
Cordlife, in response to these findings, initiated testing on the cord blood samples from one of the affected tanks. This particular tank held roughly 2.66% of the total stored CBUs. Unfortunately, MOH’s assessment indicated that these CBUs might no longer be suitable for stem cell transplantation.
As a preemptive regulatory measure, MOH decided to temporarily suspend Cordlife Singapore from accepting new cord blood, human tissues, and conducting certain tests.
Significance of the event
The significance of this event cannot be understated. When such a story makes headlines, it’s impossible not to empathize with the families directly impacted. For those who made the decision to bank their baby’s cord blood, this isn’t just a minor slip-up; it’s deeply upsetting. Cord blood is so much more than just a medical resource; for many, it’s a symbol of hope and security, safeguarding their children against future health issues.
To understand its value, it’s crucial to understand what cord blood actually is — it’s the blood that remains in the umbilical cord and placenta after a baby is born. This blood is special because it’s packed with hematopoietic stem cells, or HSCs for short. These cells are like the Swiss Army knife of cells—they can turn into red blood cells that carry oxygen, white blood cells that fight off infections, and platelets that help with healing cuts.


Source: Cordlife
Due to these unique abilities, cord blood can be used in treating over 80 different diseases, including some pretty serious ones like leukemia and lymphoma.

Source: Cordlife
Collecting cord blood is entirely safe and straightforward. Right after a baby is born, the cord blood is collected quickly and painlessly. It’s then processed and stored in super-cold freezers, kept at temperatures lower than -150 degrees Celsius.
For parents, deciding to store their child’s cord blood is a big deal. It’s not cheap, often running into thousands for the initial collection and yearly storage. Yet, the peace of mind it offers can be priceless. It’s like a safety net, holding potential for future medical breakthroughs and treatments.
That’s precisely why the situation at Cordlife, where issues arose regarding the storage temperature of cord blood, hits so hard for these families. They trusted that their child’s cord blood would be safe and potentially lifesaving. The thought that it might be compromised shakes the very foundation of that trust and the reasons they chose to bank it in the first place.
Impact on Cordlife
Apologies for going a bit off-topic, but it’s crucial to understand the bigger picture here. When we talk about a company like Cordlife in the healthcare sector, trust and a solid track record aren’t just nice-to-haves – they’re absolutely essential. These things take a lot of time and effort to build up, but unfortunately, they can be shattered in no time. From what I can see, this episode will definitely have an impact on Cordlife’s operations, both directly and indirectly.
Loss of Revenue
To get right into it, the Ministry of Health (MOH) has put a temporary hold on Cordlife Singapore’s ability to accept new cord blood, human tissues, or conduct certain tests. This suspension is set to last for up to six months. While there’s a possibility it might be lifted sooner, it could also be extended; as such, the full extent of its impact remains uncertain as the investigation, which is expected to last for another six weeks, is still ongoing. However, it’s clear that this suspension will have some kind of impact on Cordlife’s financial performance, affecting both its top and bottom lines.
Additionally, there have been comments online which mentioned that payments from existing customers have been halted till further notice. For those confirmed to be impacted, refunds for the remaining fees they’ve paid will also further add to the financial strain on the company.
So, the financial implications are not limited to just the suspension of new services; it’s also affecting the revenue from existing customers.
Loss of Trust
Beyond the immediate financial impact, the erosion of trust is a paramount concern. Following the recent incident, trust in Cordlife has reached an all-time low. The MOH’s audit has revealed some deeply unsettling findings, including the fact that Cordlife’s cryopreserved cord blood units were exposed to temperatures exceeding acceptable limits in seven out of 22 storage tanks for an extended period, dating back to November 2020—an astonishing three years. These revelations cast serious doubts on the company’s operational processes and its ability to maintain the integrity of stored cord blood units.
In addition to the temperature excursions in the tanks, the MOH uncovered significant process lapses at Cordlife, which are highly concerning:
- Cordlife’s temperature monitoring system failed to send notifications of temperature excursions in two tanks to the company’s personnel between February and June 2022.
- The six-monthly preventative maintenance for two tanks was neglected in 2022.
- Cordlife implemented a new cord blood processing method in August 2023 that was not properly validated according to an approved plan and protocol.
These findings inevitably raise substantial questions about the overall efficacy and reliability of Cordlife’s operations.
In response to SGX queries, Cordlife also disclosed yesterday (10 Dec) that despite being informed of irregular temperatures in one of its tanks in February 2023, the Board chose not to make any public announcements as they had accessed that there would be “no material impact on the financial performance of the group”. This revelation would inevitably raises questions about the integrity of the management.
In an industry where the stakes are as high as preserving potentially life-saving cord blood, the idea of taking even a 50/50 chance is unthinkable. The uncertainty generated by these lapses and their response may drive potential new customers toward competitors, as trust becomes a paramount factor in their decision-making process.
Suspension
Companies like Cordlife recognize that trust is the bedrock of this industry. This is why, in addition to the required audits and licenses from the Ministry of Health (MOH), they often go the extra mile to obtain international accreditations.
As of the time of writing, Cordlife’s FACT (Foundation for the Accreditation of Cellular Therapy) accreditation has been indefinitely suspended, at least until the ongoing MOH investigation is completed and the underlying issues are resolved. Additionally, the Association for Advancement of Blood & Biotherapies (AABB) is actively investigating the matter. While this suspension may not directly impact Cordlife’s cord blood operations, it has indirect consequences that should not be underestimated.
These accreditation bodies play a crucial role in establishing industry standards and ensuring the quality and safety of cord blood banking services. The suspension of FACT accreditation sends a clear signal that Cordlife’s practices have raised serious concerns and fall short of the high standards expected in the industry. This will undoubtedly further erode trust and confidence in the company, both among existing customers and potential clients.
In essence, the suspension of international accreditations has far-reaching implications for Cordlife’s reputation, reinforcing the importance of trust and adherence to industry standards in the healthcare sector.
Conclusion
To sum it up, the fallout from this episode will undoubtedly have both tangible and intangible effects on Cordlife’s operations. Despite any potential efforts at service recovery, the trust that has been lost is not easily regained. As the investigation continues, the full extent of the impact remains uncertain. We may see some share price recovery as more information becomes available, but at this point, it’s impossible to predict.
Nonetheless, it’s crucial to recognize that this incident has sent shockwaves throughout the entire industry. Among the private cord blood banks like Cryoviva, Stemcord, and Cordlife, Cordlife stands out as the longest-established and the largest, having served over 600,000 biological samples across Asia. If such an incident can occur within this company, it naturally raises questions about the practices of other companies in the industry.
For Cordlife, the pressing question now is how they can begin the arduous journey of rebuilding the trust they’ve lost. In an industry where trust is paramount, this task will undoubtedly be challenging, but it’s an essential step towards restoring their reputation and regaining the confidence of their clients.